Fake Rabies Vaccination in India: Abhayrab Counterfeit Vaccine Controversy
A controversy over counterfeit rabies vaccine doses in India involving Abhayrab has raised global health alerts. Learn what happened, the manufacturer’s response, public health implications, and next steps.
Rabies is a deadly viral disease that affects the central nervous system and is nearly always fatal once symptoms develop. It is most commonly transmitted through dog bites, and prompt post‑exposure vaccination is the only proven way to prevent death after exposure. India carries a significant global burden of rabies, with an estimated 18,000–20,000 human deaths annually, mostly following dog bites.
In December 2025, the Australian Technical Advisory Group on Immunisation (ATAGI) and associated health authorities issued a public health alert warning that counterfeit batches of the rabies vaccine Abhayrab had been circulating in India. The alert specifically targeted travellers who may have received the vaccine after 1 November 2023, advising them to consult healthcare providers and, if necessary, receive replacement doses.
According to these advisories, the fake vaccines:
-
Mimicked genuine products but differed in formulation, packaging, labelling, and likely lacked the correct active ingredients.
-
Could potentially leave recipients unprotected against rabies even after vaccination.
-
Were difficult to distinguish from real doses without professional verification.
While the alert was issued by Australian authorities primarily for travellers, it has drawn wide attention because rabies vaccination is a critical, life‑saving public health measure.
Which Vaccine Was Involved?
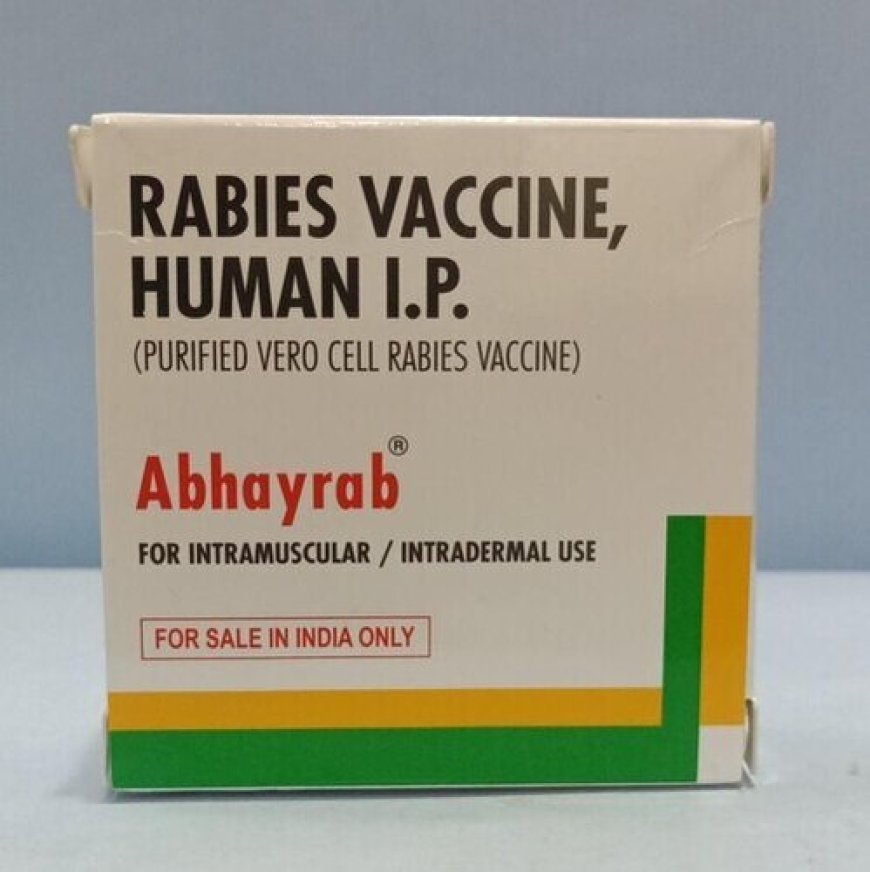
The vaccine at the centre of concern is Abhayrab, a widely used human rabies vaccine in India produced by Indian Immunologicals Limited (IIL). Abhayrab has been manufactured since 2000 and is one of the most common rabies vaccines in the country, with around 40 % market share domestically.
India’s vaccine regulatory framework requires that every batch of a vaccine is tested and released by the Central Drugs Laboratory (CDL) before it is sold or administered, a process intended to ensure safety and efficacy.
Indian Immunologicals Limited (IIL)
Indian Immunologicals Limited (IIL) has publicly responded to the international alerts, making several key points:
Isolated Incident, Not Widespread:
IIL said it had identified a specific counterfeit batch (Batch No. KA24014) circulating in the market in January 2025 due to differences in packaging and presentation. The company notified Indian regulators and law enforcement at that time, and action was taken. IIL states that this was an isolated incident and that no other counterfeit batches are present in the market now.
Counterfeits Removed:
The counterfeit batch in question is no longer available in the market, according to the company.
Safety Reassurance:
IIL has emphasised that all vaccines produced (“genuine Abhayrab”) undergo rigorous testing before release. The company said supplies through government institutions and authorised distributors remain safe, and that its quality management and pharmacovigilance systems are robust.
Dispute With Advisory Scope:
IIL described the Australian advisory as over‑cautious and misplaced in referring to circulation since 2023, arguing that the reported issues were limited and do not reflect the overall safety and quality of Abhayrab vaccines. The firm has reportedly urged foreign health authorities to reconsider parts of the advisory to avoid public confusion.
Regulatory and Public Health Actions
Public health authorities, including the Delhi Drugs Control Department, had previously raised warnings about counterfeit rabies vaccine vials circulating in cities such as Delhi, Mumbai, Ahmedabad, and Lucknow, specifically noting labelling and packaging differences. Officials highlighted that fake products may not have been stored properly or might lack sufficient active ingredients, posing a risk to individuals receiving them.
No widespread safety recalls have been issued beyond notices regarding the specific counterfeit batch. National vaccine authorities continue to monitor and ensure that vaccine quality standards are maintained.
Regulators and healthcare systems
-
Increase surveillance and monitoring of vaccine supply chains to prevent counterfeit products from entering the market.
-
Strengthen public awareness campaigns about the importance of verified vaccination and how to identify authorised vaccine sources.
-
Coordinate internationally to share verified information and avoid unnecessary public alarm while safeguarding health.
While the fake vaccine scare has raised concerns, health professionals stress that rabies vaccination remains essential and effective when obtained through official channels.
What's Your Reaction?
 Like
0
Like
0
 Dislike
0
Dislike
0
 Love
0
Love
0
 Funny
0
Funny
0
 Angry
0
Angry
0
 Sad
0
Sad
0
 Wow
0
Wow
0